Description
Semaglutide is the active peptide in Ozempic®, Wegovy®, and Rybelsus®
Tirzepatide is a dual glucose-dependent insulinotropic polypeptide (GIP) and glucagon-like peptide-1 (GLP-1) receptor agonist (RA) that was recently approved by the FDA for the treatment of type 2 diabetes. The drug is manufactured under the brand name Mounjaro™ and was approved in May 2022.
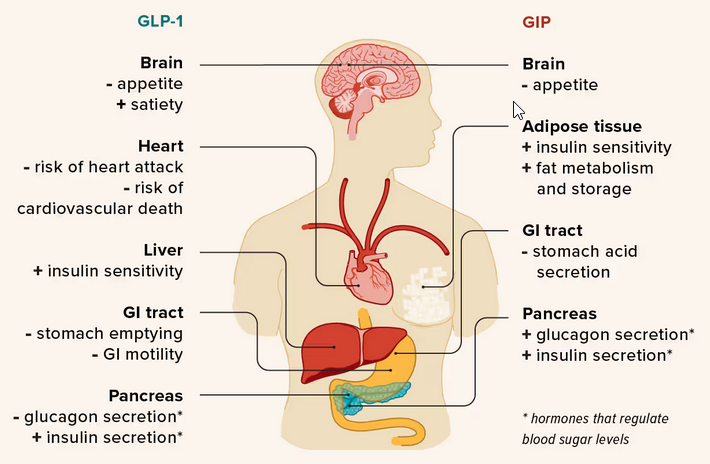
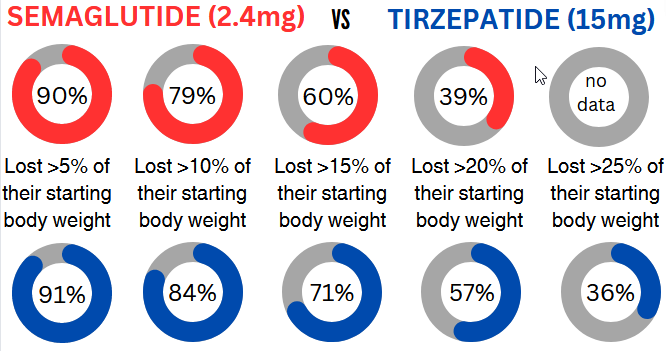
Tirzepatide and Semaglutide are both GLP-1 receptor agonists. Tirzepatide is different in that it is also a GIP receptor agonist. Here’s what this difference means for you:
The Statements and Services provided have not been evaluated by the Food and Drug Administration. These services are not intended to diagnose, treat, or cure any disease.
For More Information Click Here